In the study of chemistry, what you will learn includes the structure of matter, the composition of matter, the properties and changes of matter, and the energy that accompanies changes in matter. The properties and changes of material studied in chemistry include physical properties, which include the form and appearance of matter, as well as the chemical properties of matter that tend to change, resulting in new material. Chemistry deals with many other sciences such as Biology, Pharmacy, Geology, and Environment.
- Study and Scope of Chemistry
Have you ever thought that you live between chemicals and chemical processes? Starting from body-shaping elements and various human activities, carried out at home, at school, at work, even in space, not separated from chemical processes.
Chemistry plays a role in finding alternative materials, such as the use of fuel cells as alternative fuels, to replace depleted petroleum. Besides, chemistry also plays a role in improving quality life, by converting existing materials into more useful materials. For example: from petroleum can be converted into fuel products, paints, detergents, fertilizers, plastics, and others.
Chemistry is a natural science that learns about materials that include the structure, arrangement, nature, and changes of matter and energy that come with it.
- Study of Chemical Sciences
In the study of chemistry, you will study the structure, components, properties, and changes of matter, as well as the energy that accompanies material changes. The properties and changes of matter will be discussed in Chemistry, including physical properties and chemical properties of matter.
- Definition Material Chemicals
Matter on chemicals is anything that has mass and occupies space. Living and non-living things consist of: humans, plants, animals, water, rocks, wood, salt, and any objects around us including matter.
Matter consists of 3 kinds of forms, namely: solid, liquid, and gas. As for the characteristics:
-
- Solid: Fixed shape and volume, as long as there is no external influence.
- Liquid: The shape is always changing, according to where the volume of the liquid in place.
- Gas: Both shape and volume are not fixed and will fill all the space it occupies.
- Material Properties
We can recognize material and sort it with other materials based on their properties. The nature of matter is divided into 2, namely:
-
- Physical properties, namely the characteristics of a material that can be observed without changing the substances that make up the material. Example: color, shape, size, density, melting point, and boiling point
- Chemical properties, namely the characteristics of a substance which states whether the substance can undergo certain chemical changes. Example: flammable, and easy to rust.
According to its size, the properties of matter are divided into two, namely:
-
- Extensive properties: properties that depend on sizes, such as mass and volume.
- Intensive properties: properties that do not depend on sizes, such as physical state, color, melting point, and boiling point.
- Material Changes
In everyday life, you often see material changes. If we look closely at these objects, there are many changes. Water when boiled will turn into steam, water if cooled will turn into ice. Paper if burned will become ash, and iron if left in the air will rust. Material changes are classified into two, namely:
- Changes in physics: are changes that do not produce new matter, which change only the form and form of matter.
Contoh:
-
- Ice becomes water, and can return to ice.
- Salt dissolves, and if evaporated, will return to the original salt.
- Chemical changes: or chemical reactions are changes that produce new material. A chemical change, it is difficult to return to its original state.
Contoh:
-
- The rice turned stale
- Wood burned to ashes.
- Rusty iron
To find out if there have been chemical changes in the material, there are observable traits such as temperature changes, gas formation, or sediment formation.
- Classification of Matter in Chemistry
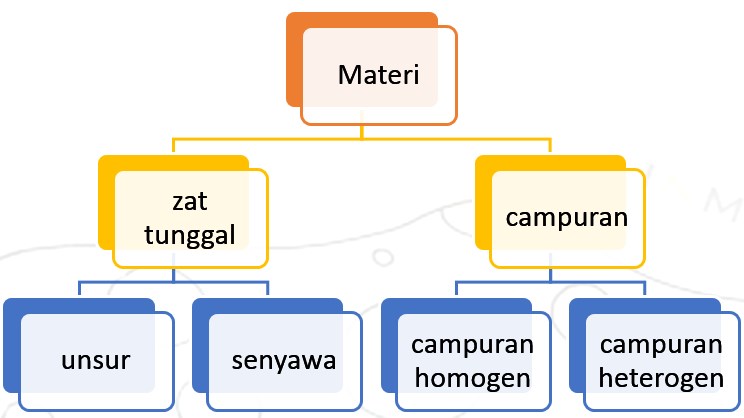
Matter in nature is of various kinds. Chemists classify them into several groups of matter, as shown in the picture below:
- Single Chemical Substance is a material that has the same properties and composition throughout it. A single substance consists of elements and compounds.
-
- An element is a single substance that cannot be broken down into other simpler substances by ordinary chemical reactions (not nuclear reactions). These elements are generally found in nature in the form of compounds. For example, sodium is found in table salt, calcium is found in limestone. Free elements, not in compound form, include copper, zinc, silver, platinum, and gold. These elements can be generally classified into metal elements and non-metal elements. Some of the metal elements are iron, copper, zinc, silver, aluminum, and so on. Some of the non-metal elements are oxygen, sodium carbon, sulfur, and so on.
- A compound is a single substance that is composed of more than one element through a chemical reaction. The properties of compounds differ from the properties of the constituent elements. For example water, sugar, salt, etc.Water is a single substance because it is only composed of one type of material. But water is not an element, because water can be broken down into several simpler materials. Water can be broken down into elements of hydrogen and oxygen..
- Compound is a material formed from the combination of two or more single substances with varying compositions. The properties and characteristics of the mixture depending on the nature of the single constituent substance.
-
- A homogeneous compound is a compound that has the same characteristics and composition throughout. A homogeneous compound is also called a solution. Solutions can be liquid, gas, or solid. For example sugar solution, salt solution, air mixture, metal mixture.
- Heterogeneous compounds are compounds where the characteristics and composition in each section are not the same. The constituents of the heterogeneous compound can easily be distinguished. Examples of cast concrete dough, ground, and vegetable soup.
- Advantages of Chemistry
By studying chemistry, we can convert natural materials into products that are more useful to meet the needs of human life, and we can understand the needs of human life, and we can understand various natural phenomena that we encounter in our daily lives, for example:
- Digestion and burning of nutrients in the body. Food comes from plants. Plant growth assimilates by chemical processes. Our bodies need carbohydrates, proteins, fats, vitamins, all of which are chemical processes that produce carbon dioxide gas, water, and energy.
- In this life, we need soap, toothpaste, textiles, cosmetics, plastics, medicines, fertilizers, pesticides, fuel, paints, cooking spices, household utensils, even various types of processed food, all of which are the result of the application of chemistry. Almost all the materials we need, more or less, either directly or indirectly experience chemical touch.
- The Role of Chemistry
Chemistry is a branch of science on which many other sciences are based. Therefore Chemistry is also called “Central Science” because of its very important role among other sciences. There is no natural science that does not depend on chemistry. Developments in medicine, pharmacy, geology, agriculture can go hand in hand with advances made in chemistry, for example in:
- Medical and Pharmaceutical
Chemistry is needed to overcome various cases, such as laboratory medical tests, manufacture of dialysis devices, manufacture of synthetic bone substitutes, teeth, and manufacture of medicines.
- Geological Sciences
Chemical science is required to research the type and composition of materials in rocks and minerals.
- Agriculture
Chemistry is used to make a wide variety of fertilizers and pesticides to increase food production.
- Industrial Sector
Chemistry plays a role in the manufacture of synthetic fibers, rayon, and nylon, to replace cotton, wool, and natural silk whose products are increasingly insufficient.
Even chemistry can also help solve social problems, such as economic, legal, artistic, and environmental problems. For example money as a medium of exchange in the economy, even the materials and manufacturing processes require chemistry. However, chemistry also requires other sciences such as mathematics, physics, and biology. Mathematics is needed to understand several parts of chemistry such as chemical calculations, reaction rates, thermochemistry, and others. Physics is needed to study, among others, thermodynamics, changes in matter, physical properties of substances, and others.
Biology is very closely related to chemistry. The association of chemistry with other sciences has spawned several branches in chemical sciences, for example, biochemistry (biology and chemistry), chemical physics (chemistry and physics), Thermochemistry (thermodynamics and chemistry), electrochemistry (electronics and chemistry), and nuclear chemistry (chemistry and nuclear).
With the basic knowledge of chemistry that we have, we understand the various natural symptoms that we encounter in life as day and can solve the problems we have.